For both mice and men, the best laid plans often require solving an unanticipated problem or two. That was my experience when I was doing research for my master's degree. I wanted to work on an insect pheromone, having been inspired by a talk by E.O. Wilson. Prof. Pipa in the Entomology Department had pointed out a 30-year-old paper demonstrating a sex pheromone in the common mealworm beetle, Tenebrio molitor, but there had been no follow-up to it. The beetle was readily available because it was raised by the millions to feed zoo animals, so I got myself a colony and got to work. Before long, I had figured out that, although I felt badly about killing them, I could extract female beetles in a solvent (e.g. ether, chloroform, alcohol, etc.), dip a glass rod in the extract, and offer the coated rod to a male beetle. The result was pure beetle pornography, for the male would mount the glass rod, extrude his genitalia, and try to copulate with the glass rod. I admit feeling vaguely guilty for fooling the males, especially as their copulatory attempts were so energetic, excited, earnest, and prolonged. Still, guilty or not, it was fun working out how much of this sex pheromone females produced, how early in life, and from what parts of their bodies this magical aroma emanated.

This last was a problem, because Tenebrio molitor was not very large, and specifying small parts of the body or glands as a source was tricky. So when, while taking a tropical entomology course in Costa Rica, I heard about a colony of large tenebrionid beetles living on a pile of bat guano in a cave, I checked it out. The beetles looked like a very large version of Tenebrio molitor and were identified as Zophobas rugipes, so I boxed up a few hundred of their larvae, applied for a USDA import permit, and took them back home to my lab in Berkeley where, being short on bat guano as I was, I fed them wheat bran. No problem. The larvae thrived and grew, squirming and wiggling in their box.
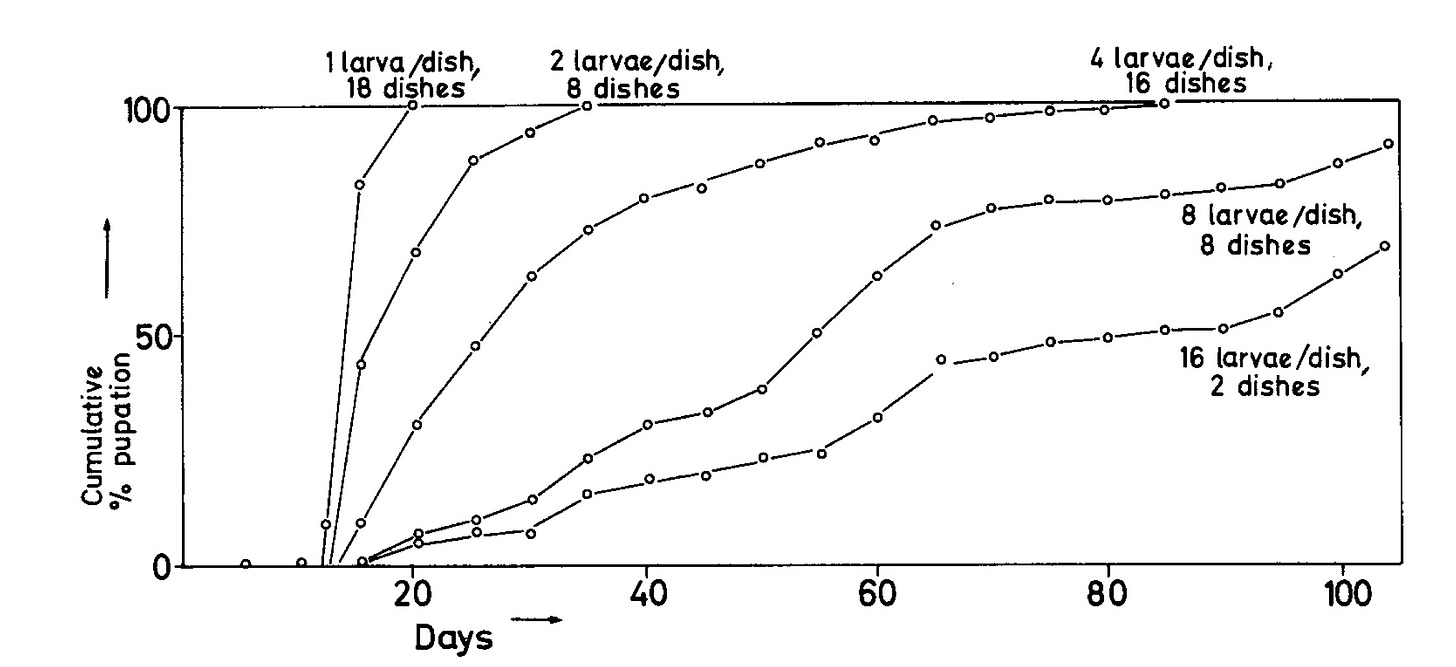
The problem arose when I needed adult beetles for the sex pheromone project, but the larvae I had imported refused to metamorphose into adults. Why not? Was it that the bran diet was missing something magical that only bat guano could provide? If so, where was I going to get bat guano in Berkeley? Or was it the wiggling, squirming, crowded conditions? I put a few larvae by themselves into Petri dishes with some bran, and sure enough, a few days later, they were curled into the pharate pupa (the first stage of metamorphosis) and a week later, they molted into pupae, followed by adult molts a couple of weeks later.

So, obviously, metamorphosis was inhibited by crowding, but crowding is a complex mixture of cues. Which cues were the crucial ones? Larvae isolated in “used” bran (mostly poop) from crowded cultures pupated quickly, so it was unlikely to be anything chemical or physical from the medium. Tactile stimulation resulting from larvae crawling over larvae seemed a likely candidate, but it could have been some other cue emanating from the larvae too. Single live larvae housed with recently killed, and therefore non-moving larvae pupated quickly, so dead larvae were not inhibitory. This left tactile stimulation as the prime suspect.
But how create the tactile stimulation of larva crawling over larva without using actual larvae? This is when a gizmo or contraption came to the rescue. In those pre-nanotechnology days, building a tiny mechanical larva was out of the question, especially on my minimal budget. My professor Clyde and I designed “The Stimulatorium”, built of cheap plastic Petri dishes, a sheet of plywood, some rubber bands cut from an inner tube on a paper-cutter, some steel rods and pulleys, and a variable speed pump motor borrowed from the lab next door. Thirty Petri dishes were fixed on the plywood, each provided with a lid on a shaft so that the lid could be rotated by means of a pulley connected to a drive shaft by one of the rubber bands. The pump motor could rotate all of these lids at the same time, and its operation was controlled by a timer.
To provide tactile stimulation to the larvae, we attached a row of bathroom stopper chain dangling from the underside of each lid, so that as the lid rotated, it dragged the stopper chain along the dish bottom. A larva in the dish would thus be tickled with every rotation of the lid. In the control dishes, the stopper chain was cut off and left in the bottom dish, providing chain, but no motion. In this way, “chain” and “tickling” were tested separately. Half the dishes were “tickled” and half were “control."

Each dish received a single larva, the motor was turned on, and the lids set into rotation. Within three days, the untickled larvae in the “control” dishes had entered pupation, but even ten days later, the “tickled” (chain) larvae had not. Dragged bathroom stopper chain had substituted for larvae crawling over larvae.

But how much tactile stimulation would it take to inhibit pupation? We reduced the number of rotations per hour and found that the fewer tickles, the larger the fraction of larvae that pupated. The larvae were measuring crowding by the frequency of tickling. No need for a census or a personal ID, stimulation frequency equated to crowding. How simple.
I had several other species of tenebrionid beetles in culture at the time, so I tested them in the Stimulatorium. Pupation was inhibited by crowding in all of them, but to very different degrees. Those that were normally very crowded during their larval periods were less sensitive, pupating at even 240 tickles per day, although at a reduced rate, while some species were far more sensitive, failing to pupate with as few as 30 tickles per day.

My contraption, The Stimulatorium, had solved the problem of providing tactile stimulation in the absence of living larvae, and had thereby answered the question of how crowding inhibited pupation. The question that remains unanswered is why did it do so? If I didn’t isolate larvae from my lab culture, they would remain larvae for up to a year, and then suffer from various geriatric ailments and die. Yet, they were capable of pupation in as little as three months after hatching from the egg. Clearly, there must be a compelling, indeed life-or-death reason why larvae do not pupate while crowded. The answer this question and its life history consequences required a long series of experiments along with another gizmo, and is the subject of a chapter to follow.
An Addendum: When we needed a large number of adults, but wanted to avoid the inconvenience of using large numbers of Petri dishes, I devised the Pupatorium, a fluorescent light grating attached to plywood and covered with a plywood lid. The single larvae placed in each grid unit smoothly transformed into pupae and adults. Here is the Pupatorium with pupae and pharate pupae.




Brings back fond memories of the Tschinkeltorium.
I'm very impressed with your ingenuity and persistence--and the math, of course. Then and now. Reminds me of one of my biology profs who, allegedly spent decades researching the stomach enzymes of certain moths. (Keep in mind this alleged activity was told to me some 60 years ago.) It was then that I realized I was in the wrong major.
Steve